Part:BBa_K2916040
Expression of RF1 in E.coli
This part is used for expression of Release Factor 1 (RF1) needed for the OnePot PURE cell-free system.
Usage and Biology
Release factors are proteins that function during the third and final step of the translation, the termination. When they recognize the termination or stop codon in an mRNA sequence, they release new peptides from the ribosome. In the standard genetic code, there are three mRNA stop codons: UAG ("amber"), UAA ("ochre"), and UGA ("opal" or "umber")
In our project we used RF1 as a part of the protein solution needed for OnePot PURE cell-free system produced with the method of gravity flow affinity chromatography, as described in the protocol we designed.
Characterization
Expression and purification of RF1
RF1 is one of the proteins we used for the OnePot PURE cell-free system. We expressed it in M15 E.coli strain using a pQE30 vector. The expression system has a T5 lac operator, RBS and a lambda t0 Terminator, enabling us to regulate the expression with IPTG.
Methods
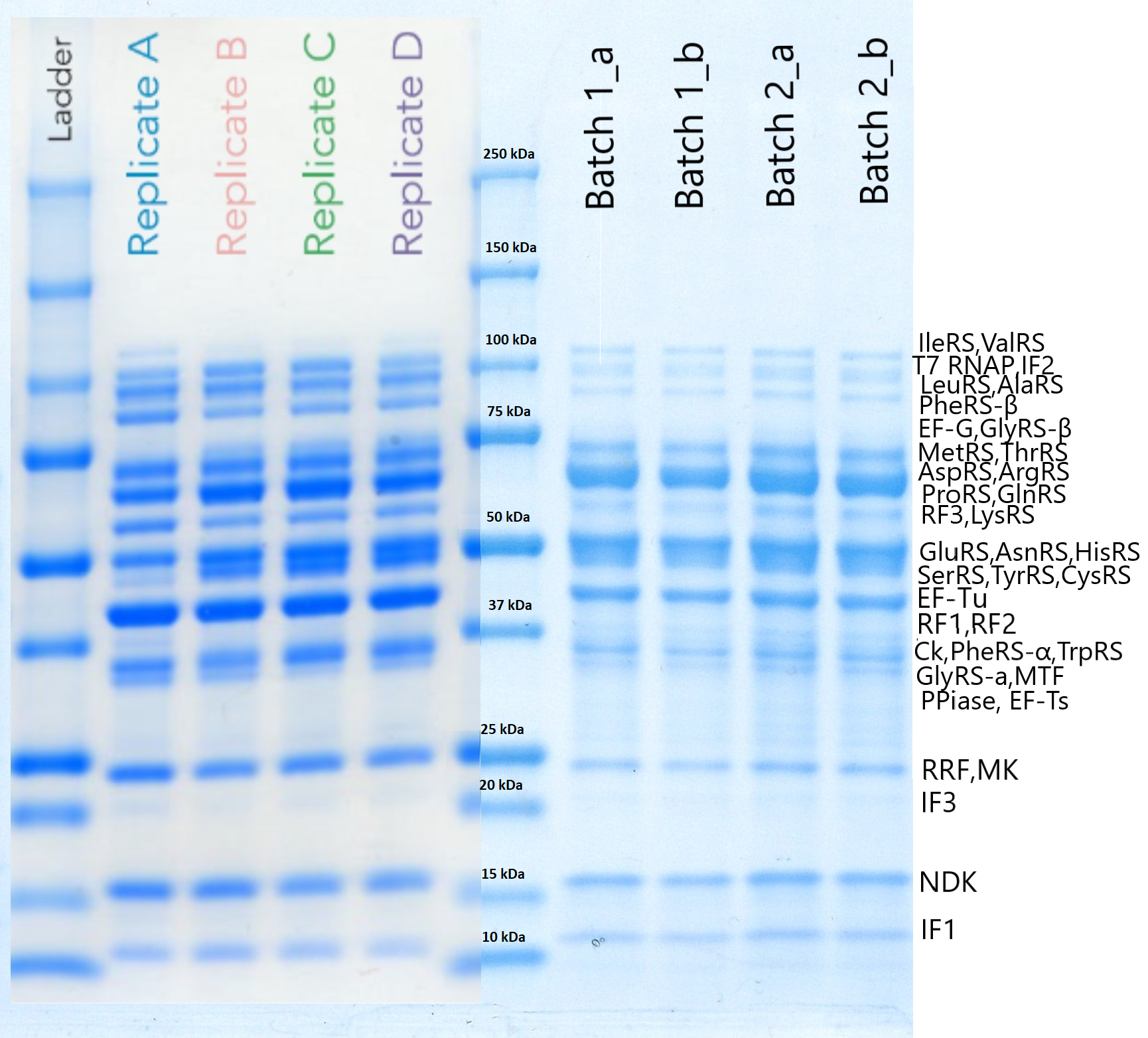
RF1 was purified using our protocol . To test if the protein was actually expressed, we performed a SDS-PAGE that is presented below. On the left side we can see the results included in the initial OnePot PURE paper (Lavickova et al, 2019) while on the right (batch1_a,b and batch2_a,b) are the solutions we produced ourselves. (The procedure we followed and the conditions of the experiment can be found here).
Conclusion
RF1 has a molecular weight of around 41kDa, but even though we cannot be absolutely sure if the band shown is only due to it, we may assume that it is expressed. To verify the existence and functionality of this protein we need to proceed with more experiments that would be mainly focused on the efficiency of the system.
OnePot PURE functionality test
To make sure that we have all the proteins in our OnePot PURE protein solution, and that they all function properly we need check if proteins can be expressed in our OnePot PURE cell-free system.
Methods
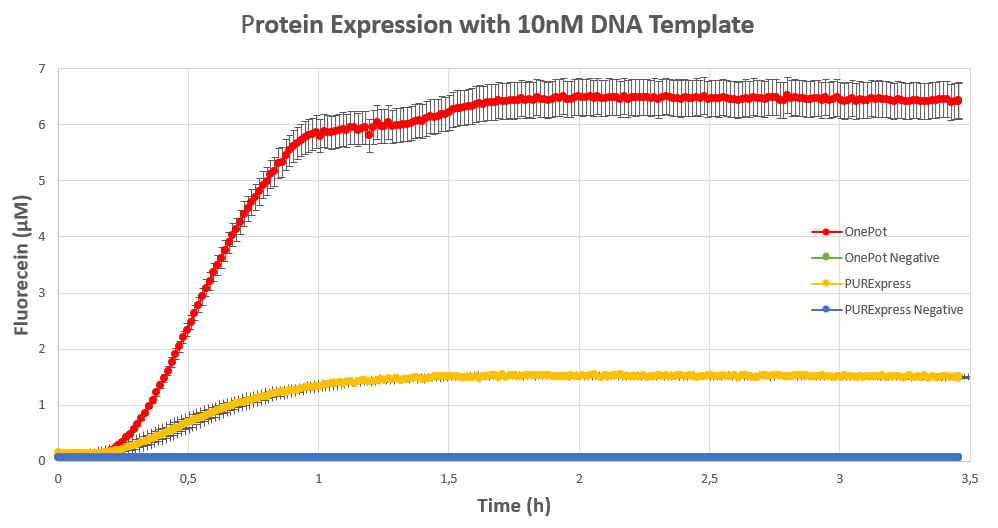
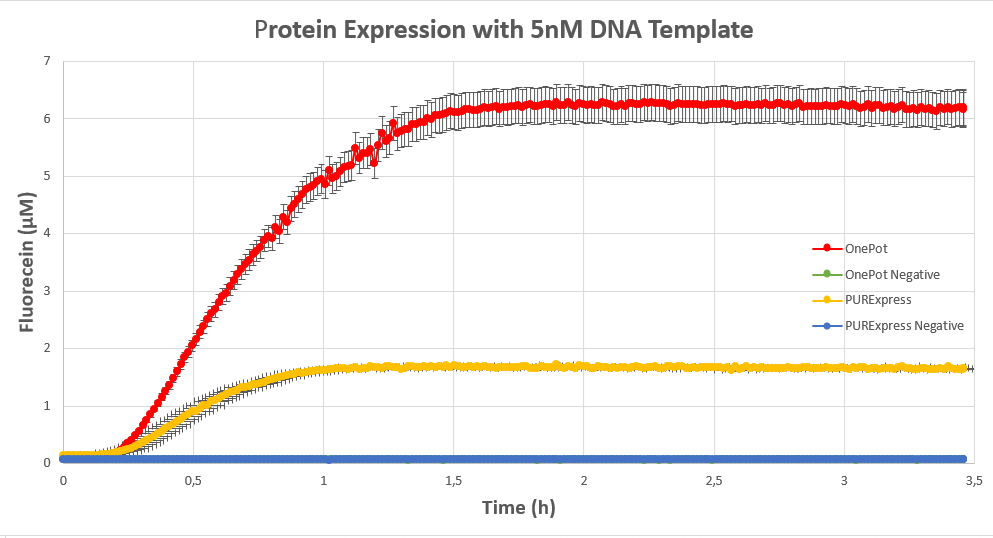
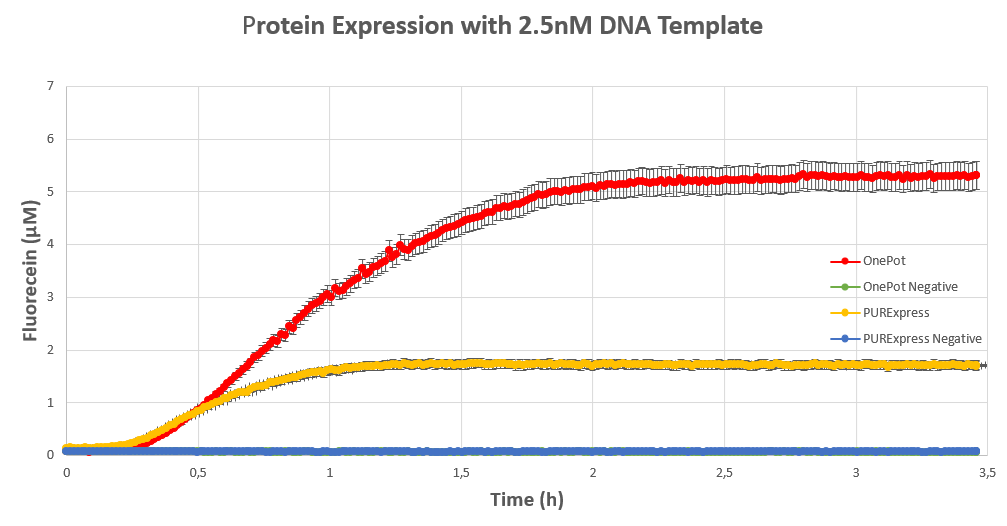
We expressed superfolding GFP following the protocol we designed in 10μl reactions, and measured the fluorescence on a plate reader at excitation wavelength of 535nm. We tested the expression using different concentrations of the sf GFP DNA template and also compared it with the fluorescence produced in PURExpress from NEB.
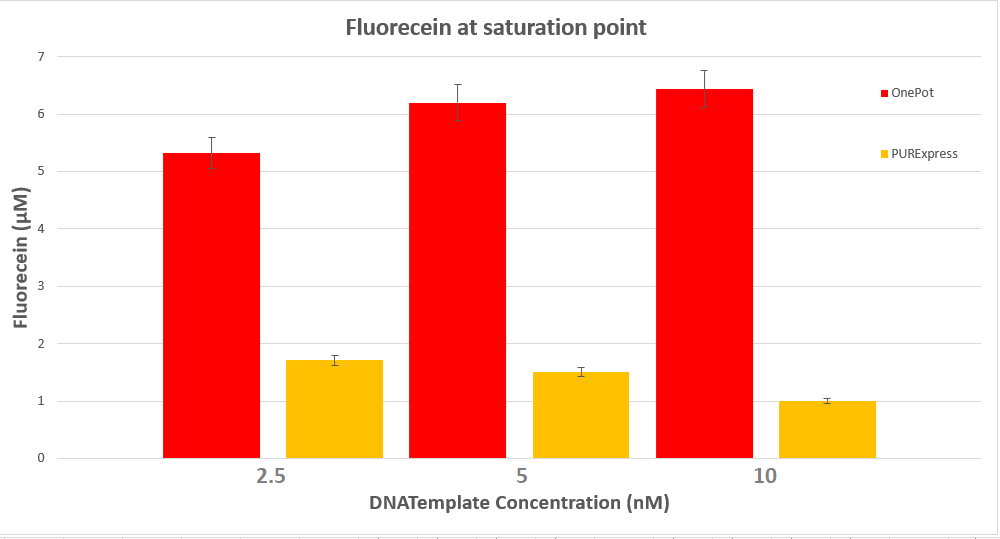
Conclusion
The expression was successful so we can confirm that RF1 exists in our protein solution and is also functioning properly.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 712
- 1000COMPATIBLE WITH RFC[1000]
| None |
